pH is used as one standard to measure the properties of water.
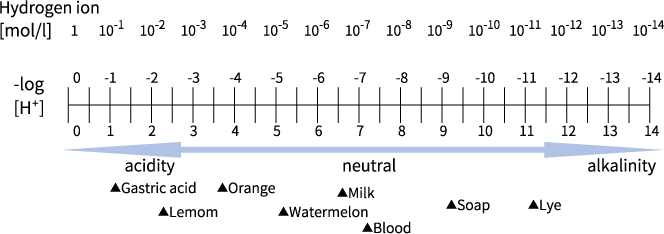
In English, it is read “pee-eych,” while in German it is read “pay-ha.” Scientifically speaking, pH expresses the amount of hydrogen ion concentration (H+) in a liquid. If the concentration of hydrogen ions (H+) in the liquid is high, it is acidic. If the concentration is low, it is basic (alkaline). This means that when the concentration of hydrogen ions is measured, the acidity, neutrality, and alkalinity, as well as the respective degree can be discovered. The pH value has a scale of 0 to 14, where 7 is neutral and any value below 7 is increasingly more acidic as it decreases in value, and where any value above 7 is alkaline and alkalinity increases as the value increases. Bitter-tasting liquids such as vinegar, juice, and lye (ash dissolved in water) are alkaline. Neutrality (pH 7) means that the hydrogen ion concentration is 10-7 (0.0000001) moles per liter. For example, in food production, food additive pH adjusters (citric acid, trisodium citrate, etc.) are widely used and they play an important role in preservation of food quality. Microorganisms have an optimal pH range for breeding, and the pH threshold of acidity and alkalinity for breeding is different for each type of microorganism. The optimal pH level for the majority of microorganisms is 7–8 (neutral or slightly alkaline), and if the pH level is outside of this range, the propagation of the microorganisms is suppressed.
For soil, the suitable pH level for raising crops is usually 6.5 (slightly acidic), but there are crops that prefer a more alkaline neutral environment, as well as some others that prefer acidity. In the manufacturing sector, pH is the standard for preventing the degradation of oil, and a pH check is essential for preventing rust on work materials and processors. The pH level of liquid coolant is usually over 9 when it’s a fresh oil. Once degradation begins the pH level decreases, but the oil should ideally be kept above pH 8.5. When it drops below pH 8, bacteria propagates and causes decomposition.
The application of pH is becoming important in a wide range of fields—from the industrial and food industries to soil and rivers.